
Advantages of the Use of Biodiesel
 Renewable fuel, obtained from vegetable oils, Used cooking oil or animal fats.
Renewable fuel, obtained from vegetable oils, Used cooking oil or animal fats. Lower emissions of contaminants: carbon monoxide, particulate matter, polycyclic aromatic hydrocarbons, aldehydes.
Lower emissions of contaminants: carbon monoxide, particulate matter, polycyclic aromatic hydrocarbons, aldehydes. No sulfur dioxide (SO2) emissions.
No sulfur dioxide (SO2) emissions. Higher flash point (100oC minimum)
Higher flash point (100oC minimum) May be blended with diesel fuel at any proportion; both fuels may be mixed during the fuel supply to vehicles
May be blended with diesel fuel at any proportion; both fuels may be mixed during the fuel supply to vehicles Excellent properties as a lubricant
Excellent properties as a lubricant It is the only alternative fuel that can be used in a conventional diesel engine, without modifications
It is the only alternative fuel that can be used in a conventional diesel engine, without modifications Used cooking oils and fat residues from meat processing may be used as raw materials
Used cooking oils and fat residues from meat processing may be used as raw materials
Tetstimonials
Products

Green Hydrogen

Green Ammonia

Biodiesel

Bio ethanol
.jpg)
Bio CNG

Fuel Cells

Hydrogen Power Generator
Features
Raw Materials for Biodiesel Production
The raw materials for biodiesel production are Used cooking oil (UCO), fresh vegetable oil, animal fats and short chain alcohols. The oils most used for worldwide biodiesel production are rapeseed (mainly in the European Union countries), soybean (Argentina and the United States of America), palm (Asian and Central American countries) and sunflower, although other oils are also used, including peanut, linseed, safflower, used vegetable oils, and also animal fats. Methanol is the most frequently used alcohol although ethanol can also be used.
Typically used Cooking Oil (UCO) is the prime source of biodiesel production in India as the obtained easily due to used for cooking heavily. Biodiesel is an alternative diesel fuel derived from vegetable oils or animal fats. The main components of vegetable oils and animal fats are triglycerides known as ester of fatty acid attached to glycerol. One of the main driving force for biodiesel widespread is the limited greenhouse gas emission (CO2 being the major one). The term used cooking oil (UCO) refers to vegetable oil that has been used in the cooking of food and which is no longer viable for its further use in food production. UCO arises from many different sources, including domestic, commercial and industrial.
Biodiesel is superior to fossil diesel fuel in terms of exhaust emissions, cetane number, flash point and lubricity characteristics, without any significant difference in heat of combustion of these fuels. Moreover, biodiesel returns about 90% more energy than the energy that is utilized to produce it. Biodiesel mixed with conventional diesel in some proportions can be used to run any existing conventional compression ignition engine and does not require any amendments to be done to the engine.
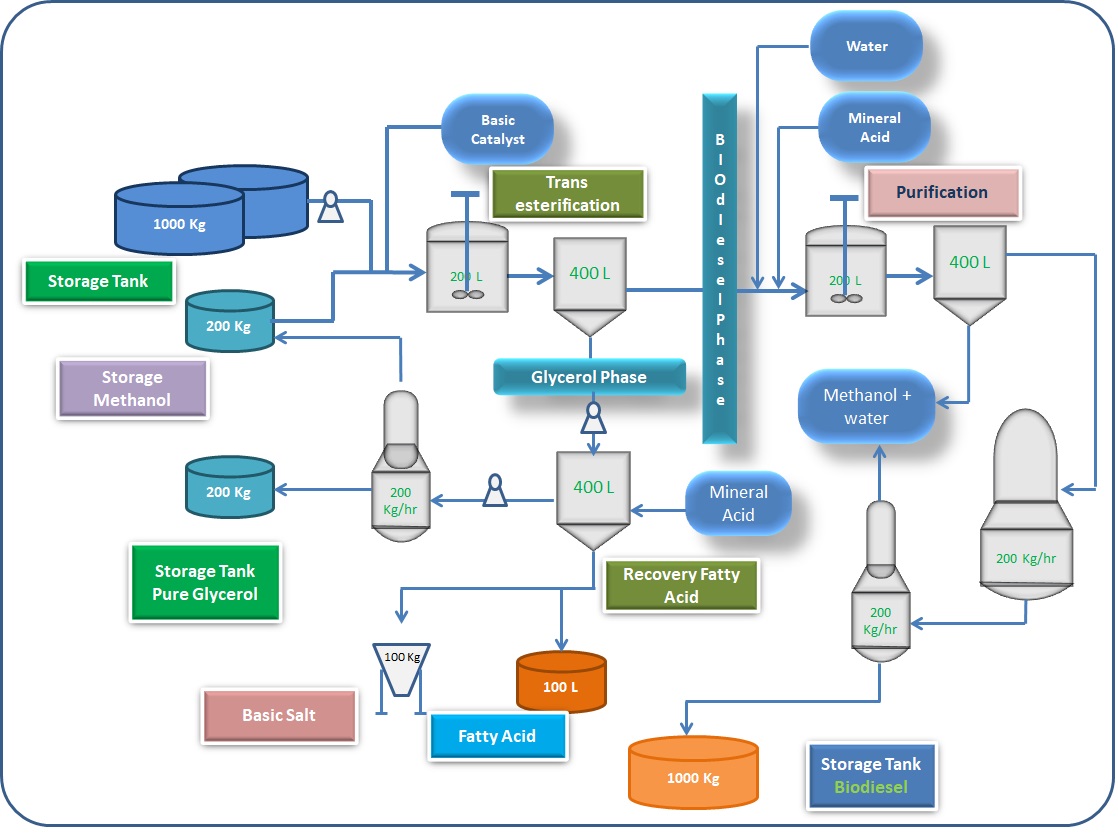
Process I
Used cooking oil (UCO) may contain particulate matter and other impurities. Such impurities are removed by filtering the waste cooking oil. Feed stock oils are usually preheated to 60oC by heat exchangers. Alcohol and acid catalysts are properly mixed in mixer before they were allowed into the esterification reactor. The mixing temperature is usually maintained at 60oC.
Catalyst mixture and preheated feedstock are allowed into the esterification reactor. Esterification reaction was carried out between 80 and 90 degree Celcius and at 1 atmospheric pressure. Products from esterification reactor were cooled to 45oC, and catalysts are removed or neutralized before moving into the settling tank to remove the methanol and water mixture. From the top of the settling tank, methanol and water mixture is removed and taken to distillation column to separate methanol from methanol water mixture and methanol is reused. The bottom product of separating vessel is taken to process II for transesterification reaction.
Process II
Catalyst and alcohol are mixed in the mixer and products formed from process are taken into the transesterification reaction column along with catalyst and alcohol mixture. Reactor temperature is usually maintained at 65oC, 1 atmospheric pressure, and 1 : 6 molar ratio of oil to alcohol. Products of transesterification reactor are moved into separator 2. From the separation tank, biodiesel and alcohol mixture is distilled to separate methanol and biodiesel.
Methanol from distillation column is recycled and reused. Biodiesel obtained is then washed with hot water and moved to separator to separate water and biodiesel. From the third separator tank, biodiesel is moved to storage tank. Bottoms of second separator section are moved to the alcohol and glycerol distillation column [41,42]. From the top of distillation column methanol is recycled. Bottom product of distillation column is a by-product.
Purification of Biodiesel:
Once the reaction is complete, the mixture of product is shifted to a separating funnel in which the mixture is allowed to settle down at room temperature or by centrifuging. The mixture gets separated into two layers in most of the cases. When ionic liquid is used as catalyst, the product formed separates into three layers based on the catalyst used. The top layer is always biodiesel and the lower layer is glycerol. This separation is based on the difference in densities. Biodiesel is collected and washed with distilled water or ethyl acetate for removing minor impurities. The washed biodiesel is heated to remove any moisture present. It may also be treated with anhydrous sodium sulphate to remove water and yield the biodiesel which is a yellow liquid.
FACTORS AFFECTING BIODIESEL PRODUCTION
The viscosity of the vegetable oil changes drastically due to process of transesterification. The removal of high viscosity component, glycerol, gives the product which has low viscosity like the fossil fuels. The biodiesel produced is completely miscible with mineral diesel in all proportions. After transesterification flash point of the biodiesel is dropped and the cetane number is improved. The yield of biodiesel in the process of transesterification is dependent on many factors such as reaction temperature, reaction time, catalyst, presence of moisture and free fatty acids (FFA), and molar ratio of alcohol and oil.
1. Temperature
Yield of biodiesel is largely affected by the Reaction temperature. For example, higher temperature increases the reaction rate and reduces the reaction time as the viscosity of oils is decreased. However, if the reaction temperature is increased beyond the optimal level, it leads to decrease of biodiesel yield, as higher reaction temperature speeds up the saponification of triglycerides [28] and facilitates the vaporisation of methanol resulting in reduced yield. So the transesterification reaction temperature should be kept below the boiling point of alcohol to prevent the evaporation of alcohol. The optimal reaction temperature may vary from 50oC to 60oC depending upon the nature of oils or fats used which should be near the boiling point of the alcohol for faster conversion.
2. Reaction time
An increase in reaction time has been found to increase fatty acid esters conversion. In the beginning the reaction is slow due to mixing and dispersal of alcohol and oil, then after some time the reaction proceeds very fast and the maximum ester conversion has been found to be attained within 90 min. Further increase in reaction time does not cause any increase in the yield of biodiesel.
3. Methanol/Ethanol (Solvent) to Oil Molar ratio
Esterification reaction is reversible so in order to shift the reaction to the right i.e to increase the yield of biodiesel, either the use of excess alcohol or the removal of one of the products from the reaction mixture is recommended.
The removal of one of the products is generally preferred for the reaction to proceed to completion. The reaction rate has been found to be highest when 100% excess methanol is used [32 and 29]. Amongst methanol, ethanol, propanol, butanol and amyl alcohol which can be used in the transesterification reaction, the methanol is used more frequently due to its low cost. Moreover, it is physically and chemically advantageous as it is polar and shortest chain alcohol. Whereas, in the transesterification process ethanol is preferred alcohol compared to methanol as it is renewable and biologically less invasive to the environment and could be derived from agricultural products. The effect of volumetric ratio of methanol/ethanol to oil has been studied and it was observed that highest biodiesel yield was nearly 99.5% at 1:6 oil/methanol. But biodiesel yield using methanol has been found to continuously increase with increase of methanol molar ratio.
4. Type and Amount of Catalyst
Biodiesel formation is also depends on the concentration of catalyst. In the transesterification process, the type and amount of catalyst needed generally depend on the quality of the oil or fat, alcohol and the method applied for the process. Sodium hydroxide (NaOH) or Potassium hydroxide (KOH) are the most commonly used catalyst for biodiesel production [28].
For pure starting materials, any type of catalyst could be used for the transesterification process. However, homogenous transesterification process is unsuitable for starting materials having high moisture and free fatty acids contents, as there is a great probability of occurrence of saponification process instead of transesterification process. The yield esters usually increase with increasing amount of catalyst as there is availability of more active sites by additions of larger amount of catalyst in the transesterification process, but it is not profitable due to cost of the catalyst itself. So it is necessary to determine the optimum amount of catalyst essential in the transesterification process
5. Mixing Intensity
Mixing is very important in the transesterification process, as oils and alcohols are not completely miscible and reaction can take place only in the interfacial region between the liquids hence transesterification reaction becomes a slow process. Therefore, sufficient mixing between these two types of reactants is essential to stimulate contact between these two reacting materials in order to promote the transesterification reactions to take place.
6. Free fatty acid and water content
The free fatty acid and water content have substantial effect on the transesterification of glycerides with alcohol using catalyst. The high free fatty acid (FFA) content more than 1% w/w, will cause the soap formation and the separation of products extremely difficult, and leads to low yield of biodiesel product [36]. In addition to this formation of gels and foams also hampers the separation of glycerol from biodiesel [28]. For example, water content in waste cooking oil speeds up the hydrolysis reaction and simultaneously reduces the amount of ester formation [37]. To overcome this difficulty, supercritical methanol method was suggested as water has been found to have less influence in supercritical methanol method [28]. So, water content should not exceed 0.5% in order to obtain 90% yield of biodiesel and it is more dangerous for an acid-catalysed reaction than base catalysed reaction.
